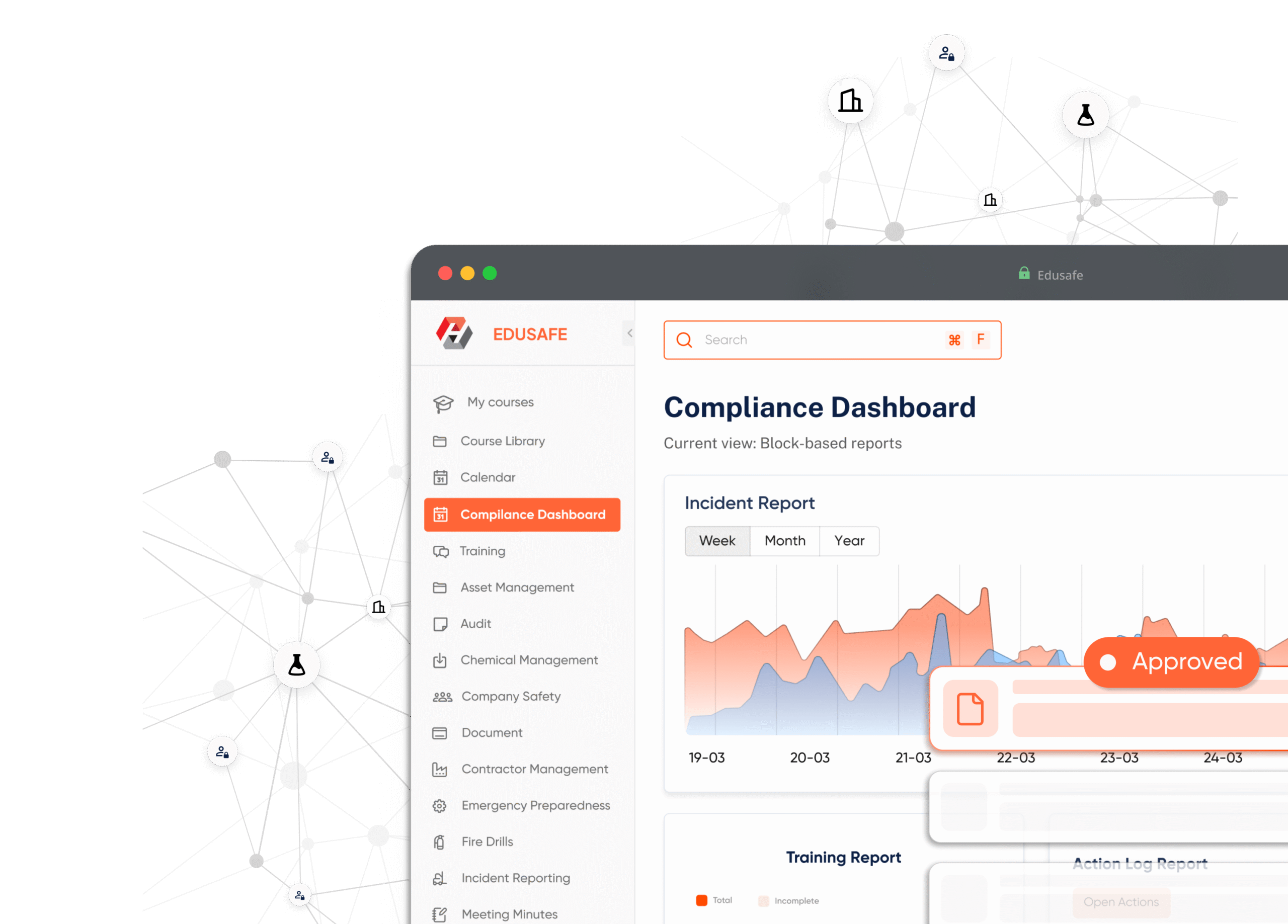
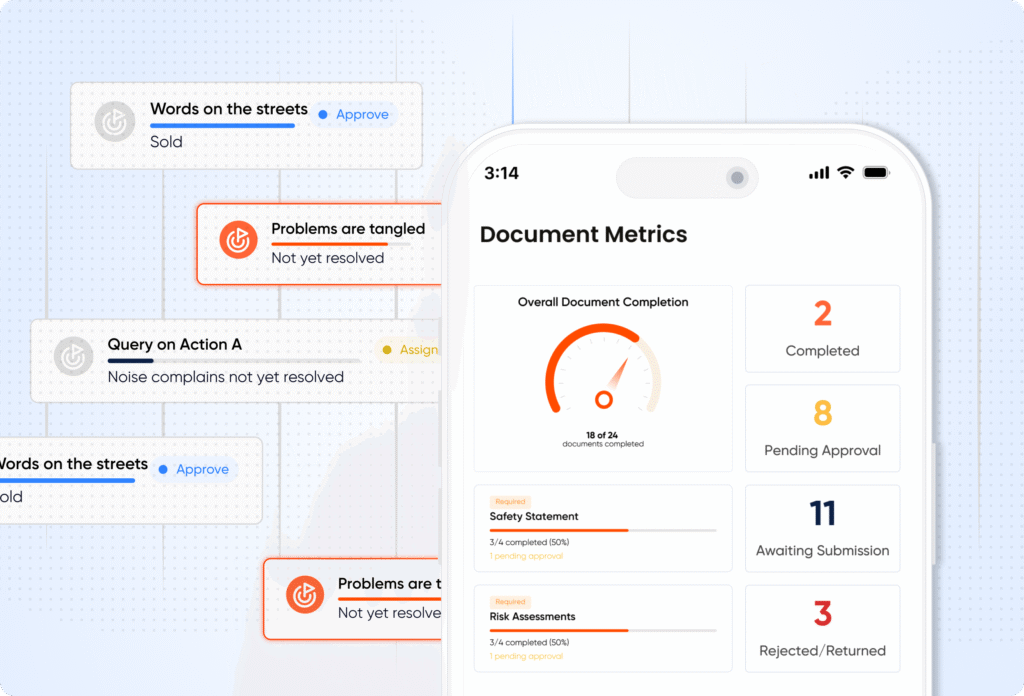
Action Log
Track corrective and preventive actions from incident to closure with full accountability and compliance proof.
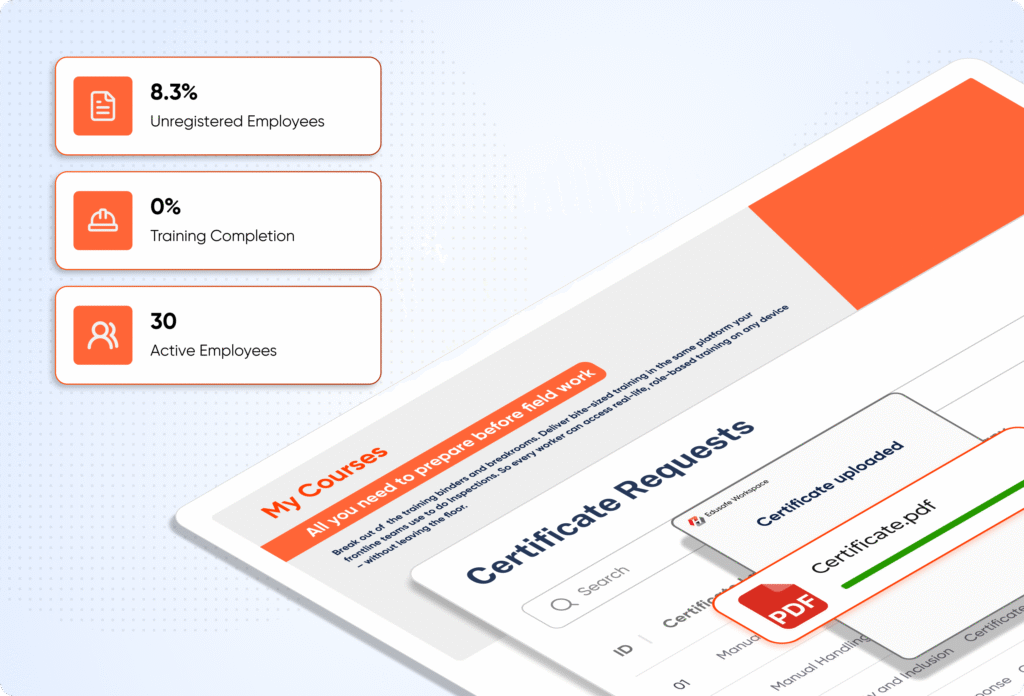
Digitise training, contractor checks, and compliance records to keep labs safe, products compliant, and inspections stress-free.


Track corrective and preventive actions from incident to closure with full accountability and compliance proof.
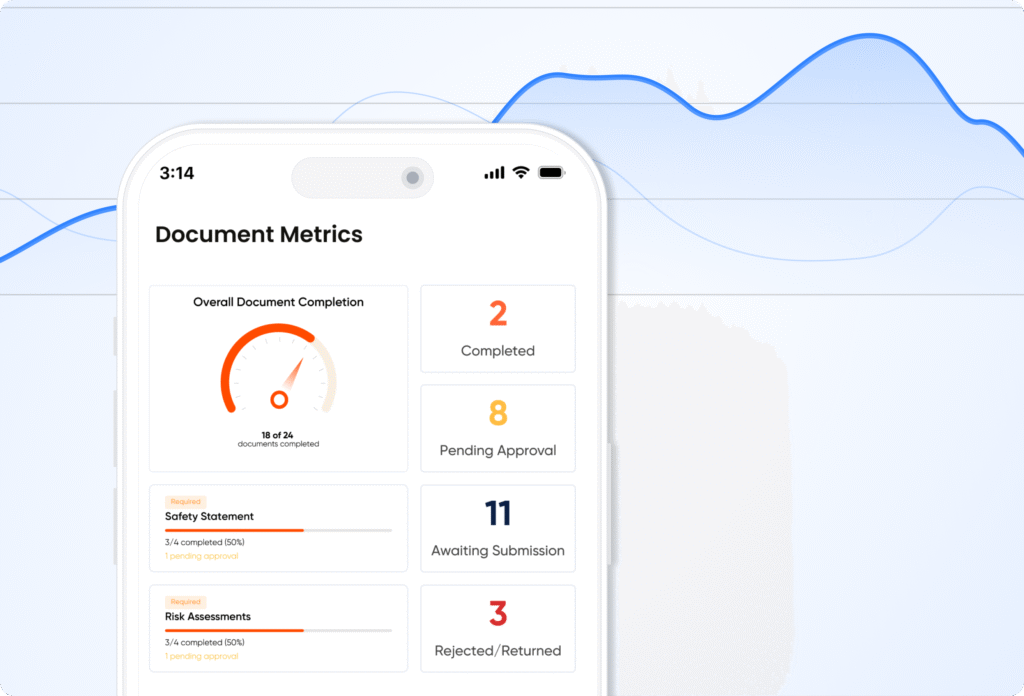
Live dashboards and analytics that give you full visibility of compliance across teams, sites, and projects.
Manage contractors across multiple sites with centralised records, structured company hierarchies, and multilingual compliance support.
Transform complex regulations into interactive, multilingual courses that scale across your workforce.
Digitise inductions, certificates, and compliance training — so employees are site-ready on day one
Identify hazards, score risks, and track mitigation with digital workflows that keep your workplace compliant. Spot hazards, score risks, and control exposure across every site aligned to HSA expectations.